Vai offline con l'app Player FM !
83. RFJC 13 – ARDS Series – DEXA-ARDS
Manage episode 436410782 series 3299598
In the penultimate episode in our ARDS Rapid Fire Journal Club Summer Series we are talking about the DEXA-ARDS trial (published in Lancet Respiratory Medicine in 2020). This trial evaluated the impact of dexamethasone in the treatment of ARDS.


Article and Reference
Today we’re discussing the DEXA-ARDS trial published in Lancet Respiratory Medicine in 2020. This trial evaluated the impact of dexamethasone on mortality and duration of mechanical ventilation for patients with ARDS.
Infographic
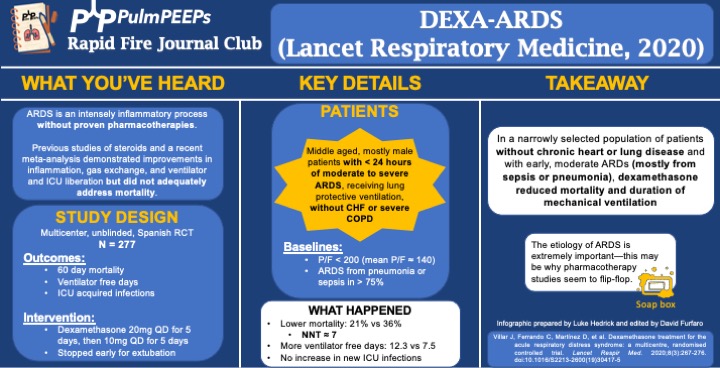
Article Notes
- DEXA-ARDS; Lancet Respiratory Medicine, 2020
- DOI:10.1016/S2213-2600(19)30417-5
- Link: https://doi.org/10.1016/s2213-2600(19)30417-5
- Background: ARDS is an intense inflammatory process without proven, specific pharmacotherapies. Previous work and a recent meta-analysis demonstrated improvements in inflammation, gas exchange, and ventilator and ICU liberation but did not adequately address mortality.
- Study Design (design, primary outcome, participants, etc)
- Design: investigator-initiated, multicenter, unblinded, randomized controlled trial in 17 academic ICUs in Spain, conducted from 3/2013 to 12/2018
- Primary Outcome
- VFD at 28d
- Secondary:
- 60d mortality
- Actual duration of ventilation in ICU survivors
- ICU acquired infections
- Participants
- Inclusion ARDS with P/F < 200 for < 24hr on LTVV
- Exclusion:
- Already receiving steroids or immunosuppression
- CHF
- Severe COPD
- DNR
- Summary: Middle aged, mostly male patients with < 24hr of moderate to severe ARDS receiving LPV without chronic heart or lung disease
- Like many ARDS trials, just over 3/4 of patients’ ARDS was caused by PNA or sepsis. Mean P/F was ~140
- Intervention/Limitations
- N = 277, stratified by center and then randomized
- Intervention: dexamethasone 20mg qd for 5d followed by 10mg qd for 5d
- Stopped early for extubation before day 10
- First dose given no more than 30 hours after P/F < 200
- Control: no placebo, just SOC
- All patients received LTVV
- Outcomes/Safety
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- As an aside, this seems to be a theme in ICU trials: massively ambitious proposed benefits during power calculations and then under-enrolling for that power calculation ultimately resulting with a point estimate that favors the intervention but is not statistically significant.
- Efficacy:
- 60d mortality: 21% vs 36%, P = 0.0047
- NNT of just < 7!
- VFD at 28d: 12.3 vs 7.5, P < 0.0001
- Actual duration of ventilation in ICU survivors: 14.2d vs 19.5d (P = 0.0009)
- 60d mortality: 21% vs 36%, P = 0.0047
- Safety:
- Hyperglycemia: 76% vs 70%, P = 0.33
- Always interesting in steroid trials when no change in glucose control is seen. This isn’t the most EBM thing I’ll ever say, but frankly I disregard this and assume steroids will cause hyperglycemia regardless of the trial results.
- ICU acquired infections: 24% vs 25%, P = 0.75
- Hyperglycemia: 76% vs 70%, P = 0.33
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- Takeaway
- In a narrowly selected population of patients without chronic heart or severe lung disease and with early, moderate ARDS (mostly from sepsis or pneumonia), dexamethasone reduced mortality and duration of mechanical ventilation.
- If time, insert soap-box about etiology of ARDS being very important (EG, flu, fungal, parasitic, mycobacterial infections)
- In a narrowly selected population of patients without chronic heart or severe lung disease and with early, moderate ARDS (mostly from sepsis or pneumonia), dexamethasone reduced mortality and duration of mechanical ventilation.
85 episodi
Manage episode 436410782 series 3299598
In the penultimate episode in our ARDS Rapid Fire Journal Club Summer Series we are talking about the DEXA-ARDS trial (published in Lancet Respiratory Medicine in 2020). This trial evaluated the impact of dexamethasone in the treatment of ARDS.


Article and Reference
Today we’re discussing the DEXA-ARDS trial published in Lancet Respiratory Medicine in 2020. This trial evaluated the impact of dexamethasone on mortality and duration of mechanical ventilation for patients with ARDS.
Infographic

Article Notes
- DEXA-ARDS; Lancet Respiratory Medicine, 2020
- DOI:10.1016/S2213-2600(19)30417-5
- Link: https://doi.org/10.1016/s2213-2600(19)30417-5
- Background: ARDS is an intense inflammatory process without proven, specific pharmacotherapies. Previous work and a recent meta-analysis demonstrated improvements in inflammation, gas exchange, and ventilator and ICU liberation but did not adequately address mortality.
- Study Design (design, primary outcome, participants, etc)
- Design: investigator-initiated, multicenter, unblinded, randomized controlled trial in 17 academic ICUs in Spain, conducted from 3/2013 to 12/2018
- Primary Outcome
- VFD at 28d
- Secondary:
- 60d mortality
- Actual duration of ventilation in ICU survivors
- ICU acquired infections
- Participants
- Inclusion ARDS with P/F < 200 for < 24hr on LTVV
- Exclusion:
- Already receiving steroids or immunosuppression
- CHF
- Severe COPD
- DNR
- Summary: Middle aged, mostly male patients with < 24hr of moderate to severe ARDS receiving LPV without chronic heart or lung disease
- Like many ARDS trials, just over 3/4 of patients’ ARDS was caused by PNA or sepsis. Mean P/F was ~140
- Intervention/Limitations
- N = 277, stratified by center and then randomized
- Intervention: dexamethasone 20mg qd for 5d followed by 10mg qd for 5d
- Stopped early for extubation before day 10
- First dose given no more than 30 hours after P/F < 200
- Control: no placebo, just SOC
- All patients received LTVV
- Outcomes/Safety
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- As an aside, this seems to be a theme in ICU trials: massively ambitious proposed benefits during power calculations and then under-enrolling for that power calculation ultimately resulting with a point estimate that favors the intervention but is not statistically significant.
- Efficacy:
- 60d mortality: 21% vs 36%, P = 0.0047
- NNT of just < 7!
- VFD at 28d: 12.3 vs 7.5, P < 0.0001
- Actual duration of ventilation in ICU survivors: 14.2d vs 19.5d (P = 0.0009)
- 60d mortality: 21% vs 36%, P = 0.0047
- Safety:
- Hyperglycemia: 76% vs 70%, P = 0.33
- Always interesting in steroid trials when no change in glucose control is seen. This isn’t the most EBM thing I’ll ever say, but frankly I disregard this and assume steroids will cause hyperglycemia regardless of the trial results.
- ICU acquired infections: 24% vs 25%, P = 0.75
- Hyperglycemia: 76% vs 70%, P = 0.33
- Power: with N = 314 (actual N = 277), 80% power to detect 2 additional VFD and 15% mortality reduction
- Takeaway
- In a narrowly selected population of patients without chronic heart or severe lung disease and with early, moderate ARDS (mostly from sepsis or pneumonia), dexamethasone reduced mortality and duration of mechanical ventilation.
- If time, insert soap-box about etiology of ARDS being very important (EG, flu, fungal, parasitic, mycobacterial infections)
- In a narrowly selected population of patients without chronic heart or severe lung disease and with early, moderate ARDS (mostly from sepsis or pneumonia), dexamethasone reduced mortality and duration of mechanical ventilation.
85 episodi
Tutti gli episodi
×Benvenuto su Player FM!
Player FM ricerca sul web podcast di alta qualità che tu possa goderti adesso. È la migliore app di podcast e funziona su Android, iPhone e web. Registrati per sincronizzare le iscrizioni su tutti i tuoi dispositivi.




